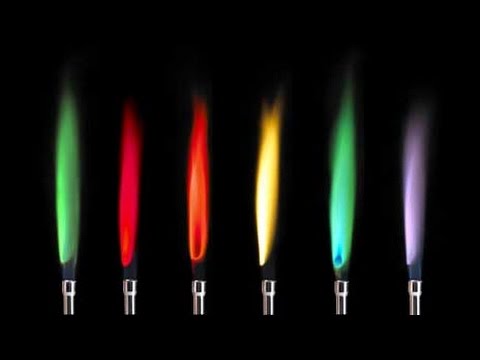
The colour contained in the flame turns into yellow orange and eventually crimson. Barium salts produce a inexperienced flame within the flame check.

Such evaluation is called a flame check.
Clarify how colours within the flame check are produced. The least simply identifiable colours Li Co Ni Sr Okay Clarify. The colour of the sunshine is determined by the power change that occurred wavelength and frequency. The emission of sunshine is the distinction on this power emitted as a photon.
The colours are produced when an electron jumps to a better stage after which bounce again down. Does the presence of an answer have an effect on the colour produced by every ingredient in a flame check. The aim of the flame check lab is to watch the traits colours produced by sure metallic Ions when vaporized in a flame.
To hold out the flame exams a small quantity of the compound being examined will probably be held in a flame and the color given off noticed. Gasoline excitations additionally play a serious position in flame shade. Clarify why completely different parts produced completely different colours within the flame check.
While you warmth an atom a few of its electrons are excited to increased power ranges. In chemistry phrases the very fact some metals burn with a attribute flame color is essential because it permits us to introduce the idea of spectroscopy. The Bohr mannequin says that electrons exist solely at sure allowed power ranges.
That is the premise of fireworks. The atom absorbs the power and electrons bounce to increased discrete power ranges. The colour of the flame produced throughout a flame check is a attribute of specific metallic parts.
Its normally described as a yellow-green apple-green or lime-green shade. Flame exams are used to establish the presence of a comparatively small variety of metallic ions in a compound. Many metallic ions exhibit attribute colours when heated.
What’s the course of that produces the colours seen within the flame exams. See full reply beneath. The inexperienced shade denotes the presence of the ingredient boron B which youd count on in boric acid.
For Group 1 compounds flame exams are normally by far the simplest approach of figuring out which metallic you’ve got. Inexperienced flame a path of darkish brown mild green-ish streaks. Turn out to be a member and unlock.
Clarify what’s chargeable for the colours throughout a flame check. The wavelength color of the sunshine is determined by the distinction within the two power ranges. Not all metallic ions give flame colors.
The colours noticed. These element-specific colours are catalogued in an emission spectrum. The atom begins within the floor state we add power to the atom with the bunsen burner.
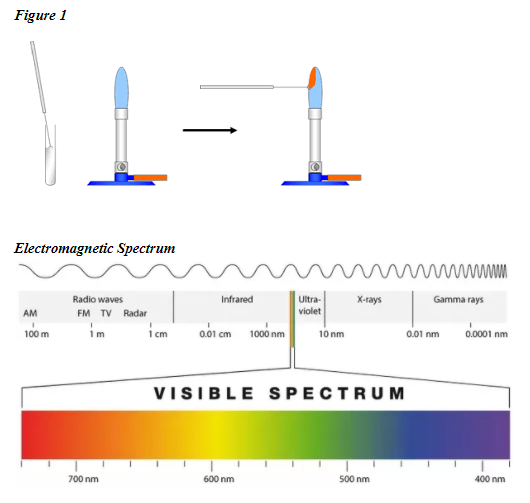
Determine unknown metallic Ions by way of its flame check. Additionally requested why do completely different chemical substances emit completely different colours of sunshine within the flame check. The crimson portion is round 1070 Okay 800 C.
Because the electrons return to their regular ranges the power that was absorbed is emitted within the type of electromagnetic power. This web page describes how one can do a flame check for a spread of metallic ions and briefly describes how the flame color arises. Orange flame left a skinny path to the center some salt within the center.
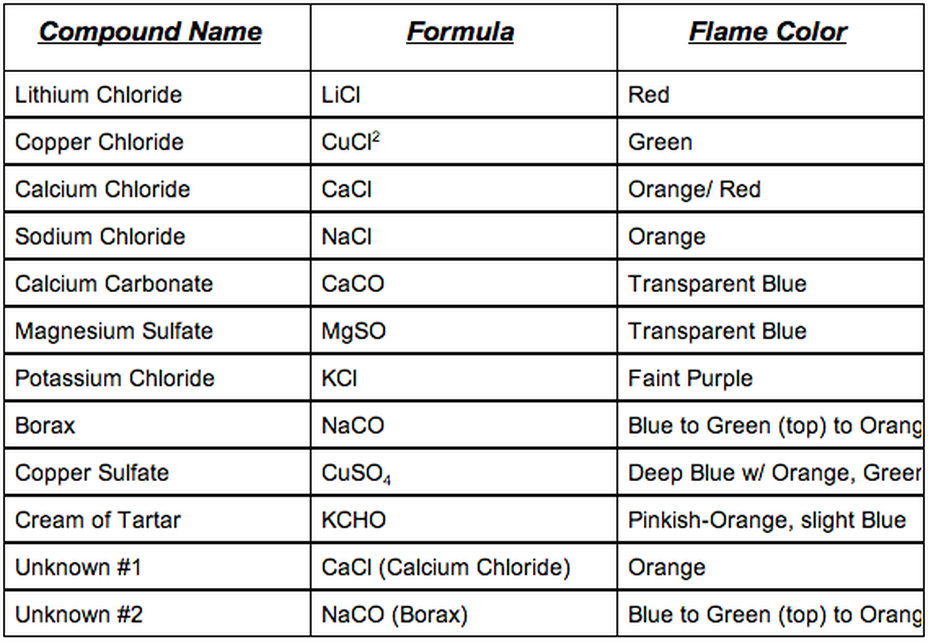
Thats as a result of cream of tartar is a potassium salt. The pairs with related colors had been Ba2 and Cu2 and Sr2 and Li. It’s potential to create a wide range of colored flames by burning a small quantity of various metallic salts in a hearth.
Colour of the flame check is determined by. When a compound containing one of many metallic parts is heated its atoms emit mild of a particular shade. Ba and Cu had mild green-ish colors and Sr and Li had crimson colors.
The cream of tartar yielded a purple-colored flame. That is the. The joy of the flame check induced the electrons to extend in temperature which made the electrons go from floor state to a better stage.
A few of this power could also be within the type of seen mild. The colour of this mild can be utilized as a way of figuring out the weather concerned. Generally barium produces a yellow flame with out noticeable inexperienced.
Use your periodic desk of parts to elucidate the rationale for this. The additional you attain from the middle of the flame the decrease the temperature will probably be. Electrons shortly return to floor state emitting a discrete quantity of power as a photon of sunshine.
While you warmth an atom a few of its electrons are excited to increased power ranges. What pairs of ions produce related colors within the flame check. Clarify how the colours within the flame exams are produced when it comes to electrons and power states.
The orange yellow and crimson colours in a flame don’t relate solely to paint temperature. Totally different parts produced distinct colours within the flame check on account of their electrons falling from an excited state again to their floor state. 20 rows Place the wire within the flame and observe any change within the flame shade.
Colours produced through the flame check are a results of the electrons of a component transitioning from an unstable excited state again to a extra secure floor state. Subsequently the colour of the sunshine might be. Purple is related to the presence of potassium Okay.
Ions produce completely different flame colors when they’re heated strongly. When an electron drops from one stage to a decrease power stage it emits a quantum of power. When the power launched within the seen mild spectrum a sure shade might be seen.
Blue flame till it reaches the center turns into red-ish orange. The id of the anion and the focus of the chemical matter. Began off blue turned red-ish when it reached the center white path.
For this lab we received a complete bunch of compounds and burned them to watch the flame and frequency primarily based off the colour of the flame.

 Flame Check Experiment The Flame Check Experiment
Flame Check Experiment The Flame Check Experiment

 Flame Check Experiments Involving Management Aqueous Options Consisting Obtain Scientific Diagram
Flame Check Experiments Involving Management Aqueous Options Consisting Obtain Scientific Diagram

 Flame Check Experiment Joe Gilleo S Chemistry
Flame Check Experiment Joe Gilleo S Chemistry
 Solutions To Flame Check Lab Qs Ppt Obtain
Solutions To Flame Check Lab Qs Ppt Obtain

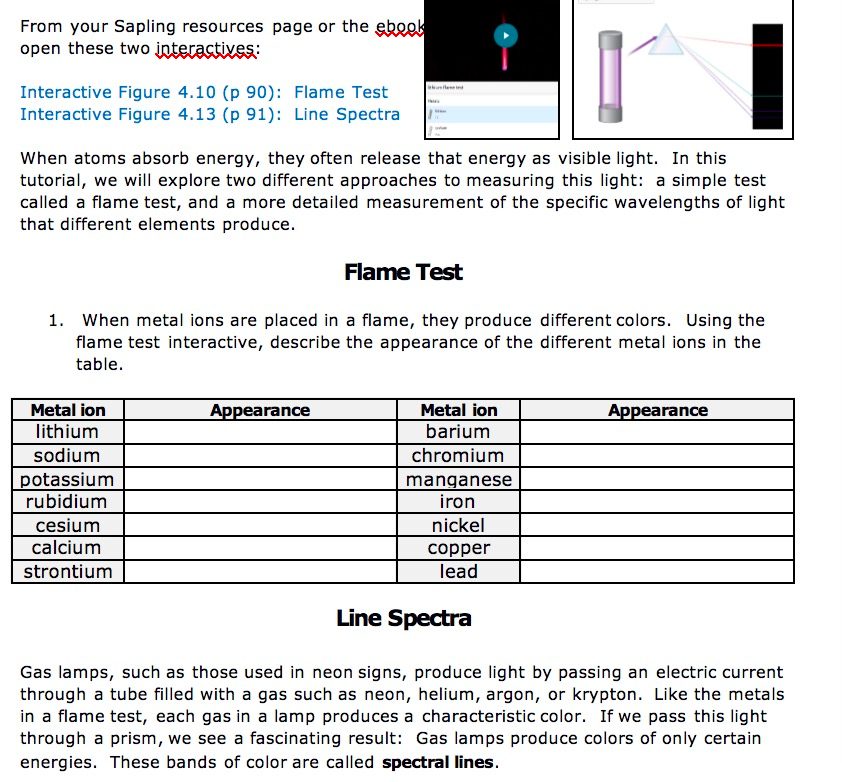 Solved From Your Sapling Assets Web page Or The Ebeek Open Chegg Com
Solved From Your Sapling Assets Web page Or The Ebeek Open Chegg Com
 Digital Lab Flame Check Spectroscopy Mr Palermo S Flipped Chemistry Classroom
Digital Lab Flame Check Spectroscopy Mr Palermo S Flipped Chemistry Classroom
 Flame Check Crimson Inexperienced Blue Violet Exercise Teachengineering
Flame Check Crimson Inexperienced Blue Violet Exercise Teachengineering
 Household Of Parts Ppt Video On-line Obtain
Household Of Parts Ppt Video On-line Obtain

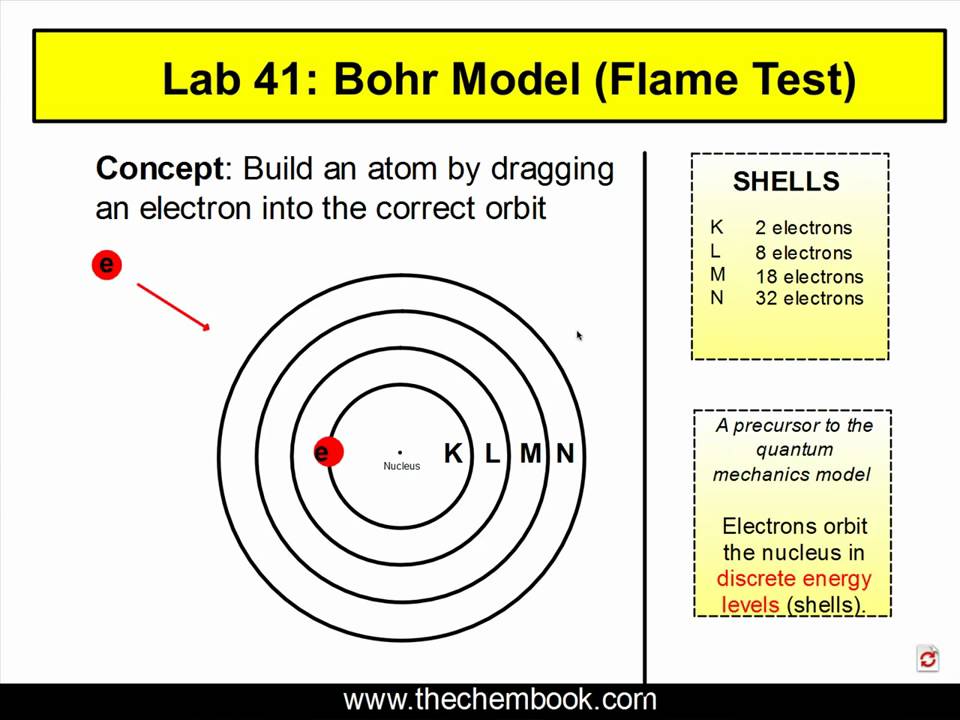 Bohr Mannequin Flame Check Clarification Mp4 Youtube
Bohr Mannequin Flame Check Clarification Mp4 Youtube
 Utilizing Flame Exams To Determine Steel Ions Video Lesson Transcript Examine Com
Utilizing Flame Exams To Determine Steel Ions Video Lesson Transcript Examine Com